The aluminium-silicon alloys are widely used in the automotive industry, due to their excellent castability, good corrosion resistance and mechanical properties, which can be further improved by eutectic Si modification with Sr, Na or Sb as modifying elements and the precipitation of nanometric Mg2Si precipitates during T6 heat treatment.
Recently, the use of secondary alloys has attracted considerable interest, as these alloys offer several advantages over conventional primary alloys, such as cheaper raw material, less energy consumption during their manufacturing and protection of resources.
However, these types of alloys usually contain high levels of alloying elements and/or impurities such as Fe, Cu and Zn which may affect adversely the microstructure, mechanical properties and corrosion resistance.
The effect of these compounds on the corrosion behaviour was studied.
Different alloys have been fabricated containing variables amounts of the target chemical elements (see table 1).
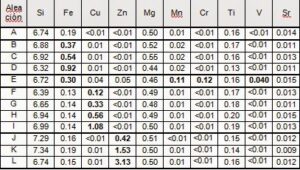
Tabla 1. Composición química de las aleaciones fabricadas (% peso)
Salt spray test have been conducted as per ASTM B177 with 336 hours of exposure. Results from macroscopic observations agree quite well with the weight loss measurements.
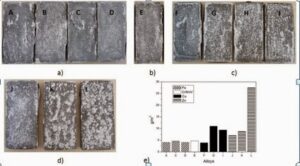
Figure 1: Surface of the test parts after 336h salt spray testing a) Reference alloy A and alloys with Fe additions, b) secondary alloy modified with Mn, Cr and V, c) alloys with Cu, d) alloys with Zn additions and e) corresponding weight loss results.
It is observed that corrosion occurs preferentially in the eutectic regions at both the Si particles and iron intermetallics interfaces (Fig. 2), while no corrosion attack was observed in the a-aluminium. In the initial stage localized corrosion occurs at the iron intermetallic interface and subsequently corrosion progresses in the eutectic Al matrix at the Si particle interface.

Figure 2: Polished corroded surfaces. a) Alloy H: Localized corrosion at the b-iron intermetallic interfaces and b) Alloy K: Progression of corrosion through the eutectic regions at the Si particles/Al interface.
The polarization curves of the reference alloy (A), alloy D and alloy E in 0,03 M NaCl solution are shown in Fig. 3a. As can be observed, all alloys exhibit a similar behavior. Additionally, the effect of the addition of Cu and Zn in the corrosion kinetics of the reference alloy was evaluated, Fig. 3b.
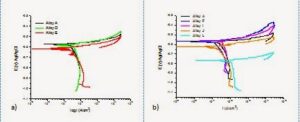
Figure 3: a) Polarization curves of the ref. alloy A, alloy D and alloy E in 0,03 M NaCl.
b) Polarization curves of the ref. alloy A, alloy F, alloy I, alloy J and L in 0,03M NaCl.
The corrosion current density is very similar in all the cases. These materials present a high susceptibility to localized attack and the pitting potential corresponds to the corrosion potential.
The addition of Fe and/or MnCrV in the concentration used in the present work appears to have no detrimental effect on the corrosion behavior with respect to the reference alloy.
The results suggest that the addition of Cu in the concentrations evaluated do not modify the corrosion behaviour of the reference alloy.
while the highest amount of Zn (3.13 wt.%) added shows a slight increase in the corrosion kinetics. This alloy revealed also in the salt spray test a significant higher weight loss than the other Cu and Zn modified alloys.
For automotive applications that require high strength, ductility and good corrosion resistance, the improvement of strength that could be obtained by addition of Cu, would be possible in concentrations of about Cu 0.5 wt.% where corrosion resistance remains still acceptable.