“This is a perfect result!”. How to get reliable results when defining analytical methods
When any chemical analysis is performed, a percentage value is usually sought as a final result. We seek how much of the element under analysis is present in the sample analyzed. Generally, this numerical value intrinsically entails all the significance of the analysis performed.
While this final figure may be the result of a simple analytical process, we must not forget that even the easiest chemical analysis is influenced by innumerable factors that can vary the final result.
In the field of metallurgical chemical analysis there are many available analytical techniques. Each one of them has different casuistic and its own instrumental parameters. Sometimes a single analysis technique is enough to get the chemical composition of the metallic material. But, usually, different techniques must be combined to get a reliable result. In order to optimize the analytical method, it is compulsory that the equipment used, and its instrumental conditions, are correctly set up.
Similarly, there are different types of materials and depending on the state of the base material and the nature of the sample, the sample preparation process varies a lot from one case to another. For instance, there is big initial difference if the morphology of the material sample is in solid state, dust or chips. The processes that derive from the preparation of the sample must be taken into consideration because that will be the first step of the analysis and it will have a direct influence when defining the most correct analytical method.
Thus, the characteristics of the sample to be analyzed, the analytical technique selected, or the instrumental conditions applied are, among others, fundamental aspects to be considered. Therefore, in order to work with scientific rigor and to obtain reliable results it is necessary to optimize the analytical method. This will facilitate a valid procedure that will allow to determine the parameter to be quantified by establishing the appropriate conditions for all the operations that are going to take part in the analytical process.
“The optimization of an analytical method implies obtaining a valid procedure to determine the parameter to be quantified, establishing the adequate conditions of all operations that are going to take part in the process.”
Let’s suppose that we have an iron base metal sample and that we want to analyze its chemical composition using the ICP-OES (Inductively Coupled Plasma – Optical Emission Spectroscopy) technique.
The mentioned sample could be a low alloyed steel, a stainless steel, an iron casting, a pig iron, a hot briquetted iron (HBI) or a direct-reduced iron (DRI).
Since all of them are iron based materials, the starting hypothesis could be that it is possible to analyze the sample using a single method. But would this be the correct choice?………… possibly not.
In this particular case, it would be more simple and “safer” to generate different specific methods depending on the material to be tested. Although all of them are iron base materials, the concentration of certain alloying elements is significantly different in each one of them (wide calibration ranges). In addition, when selecting the analysis lines that will be used to output the result, interference problems may occur.
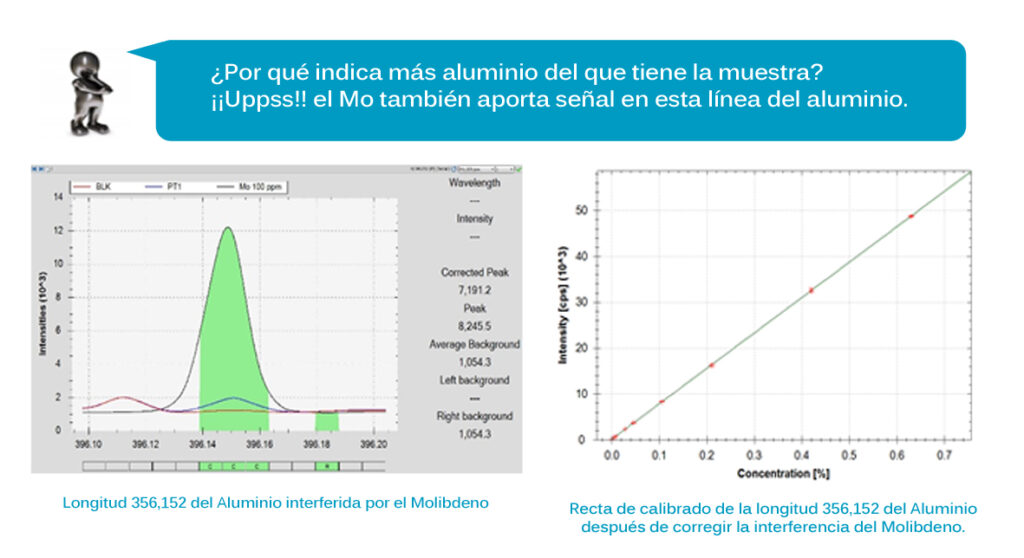
Therefore, interference among elements is also a parameter to be controlled when optimizing an analytical method.
Nowadays chemical analysis softwares allow to solve this kind of problems, by detecting these possible interferences and making possible to choose free interference lines or directly by correcting the interference level by means of mathematical formulas that will modify the calibration line and the signal of the problem-sample.
Also, optimization of an analytical method must include its validation, which is the way to ensure that this method is appropriate for the corresponding analysis, and that the results obtained are reliable and consistent.
In other words, we assure that our chemical analysis method is a robust method, despite having certain variables (the technician who performs it, the influence of the room temperature where the test is being performed, the state and conditions of the base material,… and so on).
How do we make sure that our analytical method is robust enough?
Ensuring that our analytical method is robust involves to perform a study to verify the compliance of the statistical parameters (uncertainty level of results, repeatability, accuracy, detection limit, …), including activities for internal quality control (target analysis, certified reference materials such as control samples, blind sample analysis and analysis of high, medium and low points…).
The participation in inter-comparison tests with other laboratories (same or different technique) is another significant tool to advance in the improvement of the method, because it can provide additional information that might have been initially unknown or valuable feedback that could be used to improve the analytical method itself.
On the other hand, the commercial software programs do not always consider every particular requirement from each one of the laboratories in which they are going to be used. Hence the need for each laboratory to develop their own procedures and proper applications that are capable to meet their specific demands.
Therefore, after the documentation of the steps to be followed in the preparation of the sample and the configuration of the equipment, after we have eliminated or minimized possible interferences, validated and contrasted the robustness of the method with the preliminary setting criteria (uncertainty, accuracy, linearity, etc.), after we have performed quality assurance controls to reference materials and participated in inter-comparison circuits, we can say that the result obtained by this chemical analysis method is a reliable result. “This is a perfect result!”.
Author: Arkaitz Carrasco.

