When quantitative physical-chemical tests and analyses are performed, the concept of uncertainty is usually unnoticed, giving for fact that the proportionate measure is absolutely exact and precise. It is assumed that the test or analysis process is normalized, well because international standard procedures are used for its execution, or because internal procedure validations are developed and performed by laboratories, which demonstrate their competence and rigor by carrying out these tests or analyses.
However, every test or analysis where any kind of measurement is required, even if it has been strictly carried out, has associated an uncertainty ratio (wider or narrower). This ratio depends on several factors, such as the equipment used, the reproducibility factor of the test, or the technical competence of the staff that performs the corresponding analysis
S%: 0,005 ± 0,001
Example 1. Expanded uncertainty calculated for 95% confidence interval on the result of a chemical analysis from Sulphur done by an automatic analyzer.
Though it continues being frequent to see certificates of analyses in which this uncertainty ratio is not indicated, all tests have an associates uncertainty level. This uncertainty ratio must be coherent and realistic with what the laboratory could provide for this test or this concrete analyzed parameter. In other words, if the laboratory could repeat the test of a homogeneous and stable sample (which does not alter over time), its uncertainty should cover all the variables that could affect in the measurement and the value of that parameter should always be within the range that allows its uncertainty ratio. Summarizing, the uncertainty of the test shows the range of the values where the laboratory could give the results of a concrete parameter.
S%: 0,004 – 0,006
Measurement range from Example1: The uncertainty ratio would indicate that this laboratory could certificate the Sulphur content for the same sample in this range of values.
In this regard, it is necessary to raise a slightly deeper consideration. Can the uncertainty ratio provide additional information about how this test or this analysis is performed?
There is an open discussion and certain debate at the moment trying to provide an answer to this matter. In one hand, on the basis that the uncertainties are defined by estimations, even if there are international guidelines that could help in their estimation, the truth is that even though they are justified the uncertainties have a subjective nature that, in most of the cases, try to minimize their level.
The reason that leads to this practice might give response to the question raised before. By definition, a low uncertainty ratio evidences that the equipment and the reference standard material used has an excellent resolution, that the test or the analysis has a high accuracy and that the precision and the procedure developed by the laboratory is so robust that minimize other kind of factors that could impact in the outcome of test.
When information is given in terms of fulfillment of the physical-chemical characteristics which are needed in agreement to a certain standard or specification, a low uncertainty ratio facilitates this classification.
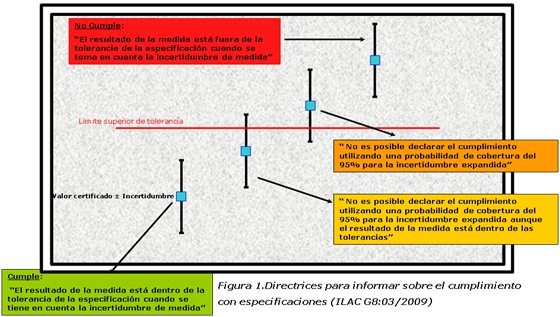 Figure 1.Directives to report on the fulfillment with specifications (ILAC G8:03/2009)
Figure 1.Directives to report on the fulfillment with specifications (ILAC G8:03/2009)
Nevertheless, there are practices that take to such an end this concept that those who know the test or the way of determining a parameter, know if these uncertainties reflect the reality, or if they are simply results of merely statistical developments.
Therefore, it is necessary to have certain caution to value the extra information that the uncertainty ratios could provide since depending on the guides used for its estimation, very diverse results could be obtained coming from the same information.
The trend during the last years is focused on having historical values, which could allow us by sight and control charts to estimate which is the uncertainty of our test.
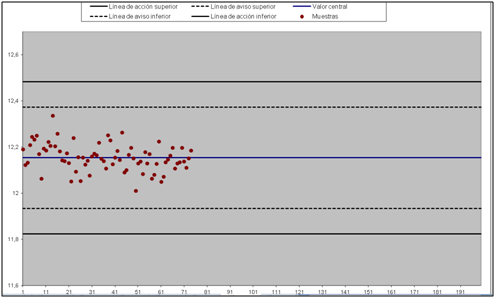
Figure 2. Control chart that gathers the historical results of a sample. In this particular case the graph represents MgO concentration levels throughout the number of days in which it has been measured.


